Live Cell Imaging
Advanced Bioimaging Modalities (2p and 3p fluorescence, P-SHG, P-THG and nano-surgery station)
Advanced (Non-linear) Imaging Microscopy Techniques for non-invasive, continuous observation of living cells and tissues.
The objectives of the project are to utilize non-invasive invasive, high-resolution, fs laser raster-scanning imaging microscopy and analysis methods through the systematic use of multiphoton excited fluorescence (2p and 3p), polarization-resolved second harmonic generation (P-SHG) and polarization-resolved third harmonic generation (P-THG), to quantitatively characterize the structure and function of living cells and tissues at video rates.
Contact Person(s):
Dr. Emmanuel Stratakis
Dr. Sotiris Psilodimitrakopoulos

Research Topics
Non-Linear Imaging Microscopy
Abstract
Critical insight and characterization of the fundamental nature of cellular and tissue function traditionally relies on detailed morphological information at the microscopic level. In recent years non-invasive invasive laser raster-scanning imaging techniques like MPEF (two-photon and three-photon), P-SHG and P-THG have emerged as new powerful high-resolution optical modalities for quantitative characterization of biological samples.
Both the above advanced optical microscopy techniques are based on minimally invasive fs laser irradiation and provide intrinsic z-sectioning due to signals nonlinear dependence on the excitation photon flux. Since the fs laser interaction is minimal, fairly nonlinear microscopy is considered as the most appropriate for live-imaging. It is based on signals originating from endogenous non-centrosymmeric molecular assemblies and lipids or on signals from exogenous species like fluorescence chromophores or nanoparticles to provide contrast. Furthermore, the infrared wavelengths used allow penetration depths (of several hundred microns into highly turbid tissues), unreachable by the common linear fluorescence or confocal microscopy.
By varying the polarization angle of the excitation field in collagen and myelin and recording the second and third harmonic intensity, respectively, we are able to produce polar diagrams whose patterns are consequently connected with the direction of the collagen and myelin molecules. Such a link is made possible through application of nonlinear optics and the derivation of equations for SHG and THG intensity that are used to fit the experimental data and infer the values of interesting parameters such as the collagen and myelin orientation and organization. This procedure allows to extract quantitative parameter values and establish a direct connection between the polarization state of the produced light and the health state of the corresponding cellular structure.
In particular, our studies on myelin, a cylindrical insulating sheath that forms around nerves, suggest that the nonlinear signal produced by mutant myelin samples differs dramatically from that of healthy ones and thus may be used as a diagnostic tool for myelin degeneration. During the theoretical treatment, myelin is approximated with a hexagonally symmetric crystal and analytical expressions are derived for the myelin third harmonic generation for both linear and circular excitation conditions.
The continuous interaction between theory and experiment within the Imaging subgroup offers an ideal means for enhancing our insight on the nonlinear phenomena occurring in organic systems under intense laser light excitation and allows for both prognostics and diagnostics of biological samples.
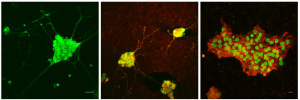
TPEF imaging in living neurons
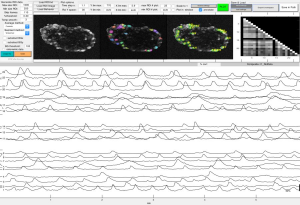
2p-Calcium Imaging of neural aggregates.
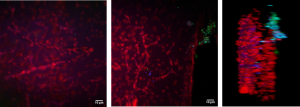
Brain slices
Simultaneous two and three photon (2p-, 3p) excited fluorescence used to visualize and characterize live and fixed brain slices, either via the use of fluorescent dyes or by excitation of autofluorescence.
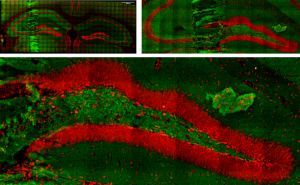
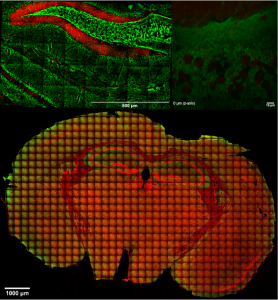

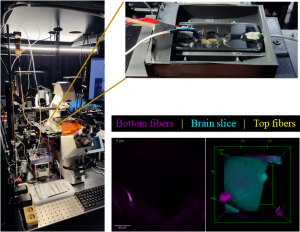
Live brain slice imaging setup
Brain slices, supplied with nutrients and oxygen in a special incubator placed at the sample plane of the microscope, are kept alive and healthy for hours during imaging, enabling the study of brain’s response to special conditions like hypoxia.
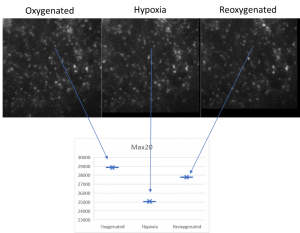
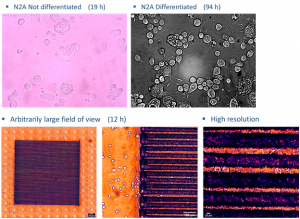
Live cell imaging
In the incubator (Ambient and living slices supporting medium temperature control, CO2 and O2 control) we can image cellular cultures for long periods, while by making collages of images we can study arbitrarily large areas with μm resolution.
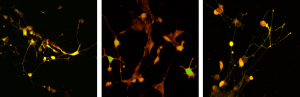
Calcium imaging
Using fluorescent dyes the electrical activity of cellular cultures and tissue is recorded via 2-PEF, providing valuable information about their dynamical connectivity and condition.
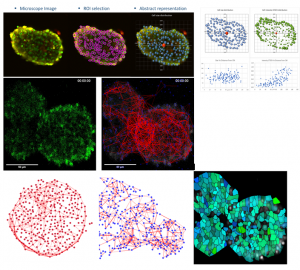
Topological characterization and determination of neuronal network electrical connectivity
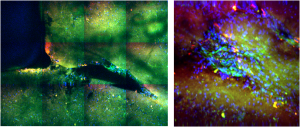
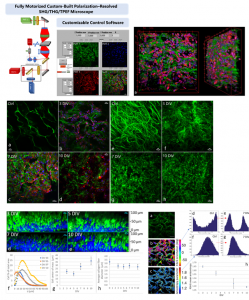
Polarization-resolved Second Harmonic Generation (P-SHG)
P-SHG has been used in our lab to visualize for the first time and optically characterize the evolution of collagen remodeling in collagen-based scaffolds seeded with live cells.
Collaborators
Prof. George Garinis
Prof. Kyriaki Sidiropoulou
Prof. Domna Garagogeos
Prof. Dimitris Tzeranis
Prof. Achilleas Gravanis
Prof. Ioannis Charalampopoulos
Dr. Katerina Gkirtzimanaki
Mrs. Constantina Georgelou
Mrs. Niki Ktena
Publications
- “Three-dimensional characterization of collagen remodeling in cell-seeded collagen scaffolds via polarization second harmonic generation,” Dionysios Xydias, Georgios Ziakas, Sotiris Psilodimitrakopoulos, Andreas Lemonis, Eleni Bagli, Theodore Fotsis, Achille Gravanis, Dimitrios S. Tzeranis, and Emmanuel Stratakis, Biomed. Opt. Express 12, 1136-1153, (2021).
- “Neural Stem Cell Delivery via Porous Collagen Scaffolds Promotes Neuronal Differentiation and Locomotion Recovery in Spinal Cord Injury” A. Kourgiantaki, D. S. Tzeranis, K. Karali, S. Psilodimitrakopoulos, M. Nikou, I. V. Yannas, E. Stratakis, K. Sidiropoulou, I. Charalampopoulos, A. Gravanis Nature Regenerative Medicine, 5, 12 (2020).
- “Ex vivo multiscale quantitation of skin biomechanics in wild-type and genetically-modified mice using multiphoton microscopy” Bancelin, B. Lynch, C. Bonod-Bidaud, G. Ducourthial, S. Psilodimitrakopoulos, P. Dokladal, J.-M. Allain, M.-C. Schanne-Klein, and F. Ruggiero, Scientific Reports 5, 17635 (2015).
- “Monitoring myosin conformational fast changes in-vivo with instantaneous single scan polarization-SHG microscopy” Psilodimitrakopoulos, D. Artigas, and P. Loza-Alvarez, Biomedical Opt. Express, 5, 4362 (2014).
- “Quantitative imaging of microtubule alteration as an early marker of axonal degeneration after ischemia in neurons” Psilodimitrakopoulos, V. Petegnief, N. de Vera, O. Hernandez, D. Artigas, A. M. Planas, and P. Loza-Alvarez, Biophys. J., 104, 968 (2013).
- “Femtosecond laser axotomy in Caenorhabditis elegans, and collateral damage assessment using a combination of linear and nonlinear imaging techniques” I.C.O. Santos, M. Mathew, O. E. Olarte, S. Psilodimitrakopoulos, and Pablo Loza-Alvarez, PLoS ONE, 8, e58600 (2013).
Project Members
Dr. Emmanuel Stratakis
Dr. Sotiris Psilodimitrakopoulos
Dr. Leonidas Mouchliadis
Mr. Dionysios Xydias
Mrs. Maria Kefalogianni
Mrs. Lida Vagiaki
Mr. Andreas Lemonis
Mr. Christos Ntoulias
Dr. Lina Papadimitriou
Dr. Anthi Ranella
Dr. Evi Kanatzikidou
Dr. Ritsa Babaliari
Mrs. Fereniki Moschogiannaki

