Synthesis of Perovksite Nanocrystals and Related Applications
Synthesis of perovskite nanocrystals and related applications
The high demand for smart and portable devices in everyday life activities creates a great need for energy supply and sensors, are driving the scientific community to explore novel materials for efficient energy conversion, storage and sensing elements. Perovskites, a prominent category of materials, including metal halides and perovskite oxides hold a significant role among energy materials but also as sensing elements, which can effectively replace the conventional ones. Nanostructuring of the perovskites due to their reduced dimensions are advantageous in offering large surface area, extensive porous structures, controlled transport and charge-carrier mobility, strong absorption and photoluminescence and confinement effects. These features together with the unique tunability in composition, shape and functionalities make the perovskite nanocrystals, uniquely suited for efficient energy-related applications such as photovoltaics, catalysts, thermoelectrics, batteries, supercapacitors and hydrogen storage systems, as well as replacing common metal oxides as sensing elements.

The research of our group is mainly focus on the development of all-inorganic metal halide perovskites. These semiconducting nanocrystals exhibit a completely different behavior from their bulk materials of the same stoichiometry due to quantum confinement effects. Optical/electronic properties can be affected in an unexpected way from the size/shape particle modifications. Their fascinating properties together with their colloidal form make these materials ideal candidates for application in energy-related applications.
Wet chemistry approaches have been used for the synthesis of perovskite nanostructures in centrosymmetric or non-centrosymmetric morphologies (spherical particles, nanoplatelets, nanosheets or more complex structures). These approaches have been carefully selected because they can allow developing finely size-, shape-, and composition- tailored nanocrystals by careful regulation of thermodynamically/kinetically driven growth processes in the liquid phase.
The perovskite nanocrystals synthesized with wet chemistry methods are well crystalline and dispersed in organic solvents as they covered with organic ligands. They exhibit very high quantum yields, narrow line width and high stability. Their emission could cover the whole visible range by modifying their composition, morphology and size.
Metal halide perovskite nanostructures covered by organic molecules or free of ligands have been grown in colloidal solutions and then deposited on substrate or grown directly on it. Their structural features together with their physical properties have been carefully investigated and the impact of the structure, morphology, and composition on energy device performance have been evaluated.
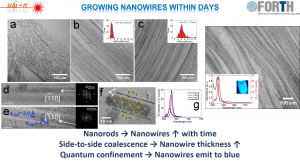
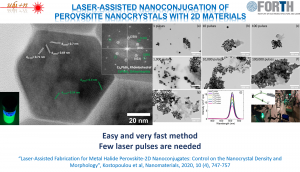
A facile and rapid photo-induced process to conjugate graphene-based materials with metal-halide perovskite nanocrystals has been explored. A small number of laser pulses is sufficient to decorate the 2-dimensional (2D) flakes with metal-halide nanocrystals without affecting their primary morphology. At the same time, the density of anchored nanocrystals can be finely tuned by the number of irradiation pulses. This facile and rapid room temperature method provides unique opportunities for the design and development of hybrid perovskite-2D materials nanoconjugates; exhibiting synergetic functionality by combining nanocrystals of different morphologies and chemical phases with various 2D materials.
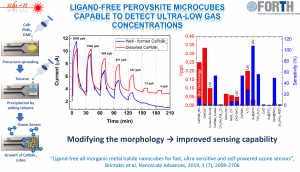
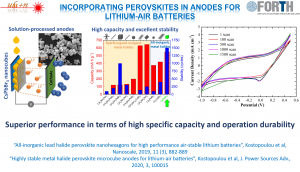
The unique features of these materials together with the unique tunability in composition, shape and functionalities make these nanocrystals, uniquely suited for varied applications. The potential utilization of these nanocrystals in energy storage as anode materials in Li-air batteries or in gas sensing application as active materials to detect ozone or hydrogen.

Publications
[1] Investigation of Si-Coated Multiwalled Carbon Nanotubes as Potential Electrodes for Multivalent Metal-Ion Electrochemical Energy Storage Systems, S. Daskalakis, A. Kostopoulou, K. Brintakis, E. Stratakis, V. Prasad Prasadam, K. Menguelti, N. Bahlawane, D. Vernardou, The Journal of Physical Chemistry C 2023, 127 (27), 13364-13379, https://doi.org/10.1021/acs.jpcc.3c02871
[2] Two-dimensional metal halide perovskites and their heterostructures: from synthesis to applications, A. Kostopoulou, I. Konidakis, E. Stratakis, Nanophotonics 2023, 12 (9), 1643-1710,https://doi.org/10.1515/nanoph-2022-0797
[3] Highly sensitive ozone and hydrogen sensors based on perovskite microcrystals directly grown on electrodes, A. Argyrou, K. Brintakis, A. Kostopoulou, E. Gagaoudakis, I. Demeridou, V. Binas, G. Kiriakidis, E. Stratakis, Journal of Materiomics 2022, 8 (2), 446-453, https://doi.org/10.1016/j.jmat.2021.07.002
[4] Laser-induced morphological and structural changes of cesium lead bromide nanocrystals, A. Kostopoulou, K. Brintakis, M. Sygletou, K. Savva, N. Livakas, M. A. Pantelaiou, Z. Dang, A. Lappas, L. Manna, E. Stratakis, Nanomaterials 2022, 12 (4), 703,
https://doi.org/10.3390/nano12040703
[5] Highly stable metal halide perovskite microcube anodes for lithium-air batteries, A. Kostopoulou, D. Vernardou, D. Makri, K. Brintakis, K. Savva, E. Stratakis, Journal of Power Sources Advances 2020, 3, 100015, https://doi.org/10.1016/j.powera.2020.100015
[6] Laser-Assisted Fabrication for Metal Halide Perovskite-2D Nanoconjugates: Control on the Nanocrystal Density and Morphology, A. Kostopoulou, K. Brintakis, E. Serpetzoglou, E. Stratakis, , Nanomaterials 2020, 10 (4), 747,
https://doi.org/10.3390/nano10040747
[7] Highly luminescent and ultrastable cesium lead bromide perovskite patterns generated in phosphate glass matrices, I. Konidakis, K. Brintakis, A. Kostopoulou, I. Demeridou, P. Kavatzikidou, E. Stratakis, Nanoscale, 2020,12, 13697-13707,
https://doi.org/10.1039/D0NR03254A
[8] Perovskite nanocrystals for energy conversion and storage, A. Kostopoulou, K. Brintakis, NK. Nasikas, E. Stratakis, Nanophotonics 2019, 8, 1607-1640, https://doi.org/10.1515/nanoph-2019-0119
[9] The role of ligands in the chemical synthesis and applications of inorganic nanoparticles, A. Heuer-Jungemann, N. Feliu, I. Bakaimi, M. Hamaly, A. Alkilany, I. Chakraborty, A. Masood, M. F. Casula, A. Kostopoulou, E. Oh, K. Susumu, M. H. Stewart, I. L. Medintz, E. Stratakis, W. J. Parak, A. G. Kanaras, Chemical Reviews 2019, 119, 4819-4880 (Front Cover),
https://doi.org/10.1021/acs.chemrev.8b00733
[10] Ligand-free all-inorganic metal halide nanocubes for fast, ultra-sensitive and self-powered ozone sensors, K. Brintakis, E. Gagaoudakis, A. Kostopoulou, V. Faka, K. Argyrou, V. Binas, G. Kiriakidis, E. Stratakis, Nanoscale Adv. 2019, 1, 2699-2706,
https://doi.org/10.1039/C9NA00219G
[11] All-inorganic lead halide perovskite nanohexagons for high performance air-stable lithium batteries, A. Kostopoulou, D. Vernardou, K. Savva, E. Stratakis, Nanoscale 2019, 11, 882-889, https://doi.org/10.1039/C8NR10009H
[12] Unveiling the Structure of MoSx Nanocrystals Produced upon Laser Fragmentation of MoS2 Platelets, K. Alexaki, A. Kostopoulou, M. Sygletou, G. Kenanakis, E. Stratakis, ACS Omega 2018, 3 (12), 16728-16734, https://doi.org/10.1021/acsomega.8b01390
[13] Perovskite nanostructures for photovoltaic and energy storage devices, A. Kostopoulou, E. Kymakis, E. Stratakis, J. Mater. Chem. A, 2018, 6, 9765-9798, https://doi.org/10.1039/C8TA01964A
[14] Anion Exchange in Inorganic Perovskite Nanocrystal Polymer Composites, M. Sygletou, M.E. Kyrazi, A.G. Kanaras, E. Stratakis, Chemical Science 2018, 9, 8121-8126, https://doi.org/10.1039/C8SC02830C
[15] Low-temperature Benchtop-synthesis of All-inorganic Perovskite Nanowires, A. Kostopoulou, M. Sygletou, K. Brintakis, A. Lappas, E. Stratakis, Nanoscale, 2017, 9, 18202-18207, https://doi.org/10.1039/C7NR06404G
Project Members:
- Dr Emmanuel Stratakis (PI, Research Director)
- Dr Athanasia Kostopoulou (Co-PI and subgroup leader, Research Associate)
- Dr Konstantinos Brintakis (Co-PI, Research Associate)
- Dr Konstantina Alexaki (Research Associate)
- Mrs Aikaterini Argyrou (PhD Candidate)
- Mrs Maria Syskaki (Master Student)
- Mr Nikos Kavousanos (Master Student in collaboration with HMU)
Alumni
- Dr Kyriaki Savva
- Dr Maria Sygletou
- Mrs Michaila-Akathi Pantelaiou
- Mr Nikolaos Livakas
- Mrs Serpil Kiokekli
- Mrs Markella Splinaki
- Mrs Maria Peraki
Collaborators
Prof Liberato Manna (Istituto Italiano Di Tecnologia)
Dr Joanathan Beauchamp (IVV Fraunhofer)
Dr. Dimitra Vernardou (Hellenic Mediterranean University, HMU)
Dr. George Kioseoglou (University of Crete)
Dr. Emmanuel Gagaoudakis (IESL-FORTH)
Dr. Vasileios Binas (IESL-FORTH)
Prof George Kyriakidis (IESL-FORTH)
Dr. Konstantinos Petridis (HMU)
Dr. Alexandros Lappas (IESL-FORTH)
Dr. Mirko Prato (Istituto Italiano Di Tecnologia)
Dr. Giorgio Divitini (Istituto Italiano Di Tecnologia)
Dr Iurii Ivanov (Istituto Italiano Di Tecnologia)
Prof Antonios Kanaras (University of Southampton)